12 Facts About the Pancreas
By Emily Petsko
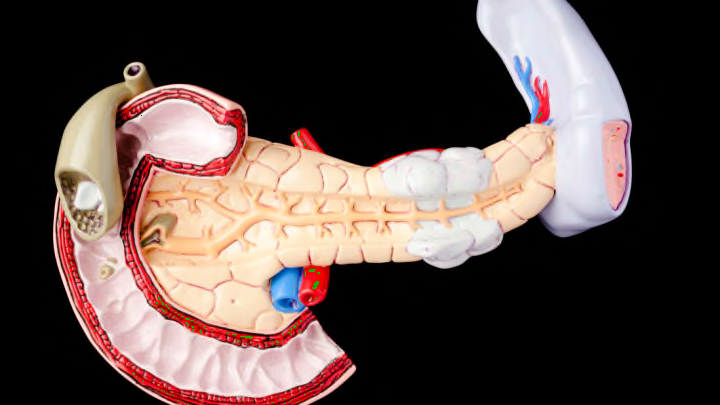
You could live without your pancreas, but it wouldn’t be easy. For one, you would need to give yourself insulin shots on a daily basis because you would develop diabetes. A helping of enzyme pills would also be needed to help you digest food. It's clear that the 6-inch-long pancreas, located behind your stomach, has crucial functions—and that's why diseases like pancreatic cancer and pancreatitis are often so devastating. Here are a few other important facts to know about the pancreas.
1. Pancreas means “all flesh” in Greek.
Around 300 BCE, a surgeon in ancient Greece named Herophilus became the first person to formally describe the pancreas as a gland. However, the organ didn’t get its name until about 400 years later, when another Greek surgeon and anatomist named Ruphos dubbed it the pankreas, meaning “all flesh”—possibly because of its lack of bone or cartilage. (The plural of pancreas, by the way, is pancreata or pancreases.) Later, in the 16th century, people started referring to a dish of cooked calf or lamb pancreas as “sweetbreads.” That name possibly stems from bræd, the Old English word for “flesh.”
2. The pancreas has a head and a tail.
The pancreas has four main parts: the head, neck, body, and tail. The widest part is the head, which is attached to the first part of the small intestine, known as the duodenum. In cases where a pancreatic tumor is present, the head is usually the part that’s affected. However, according to one study from 2008, people with tumors in the body or tail of the pancreas had lower survival rates than those with cancer in the head of the pancreas.
3. The man who discovered the pancreatic duct may have been murdered for his work.
The pancreatic duct is a tiny tube that runs the length of the pancreas and carries digestive juices to the duodenum. Although the ancient Greeks knew about the pancreas, its function and anatomy weren’t fully understood for centuries. That started to change in 1642, when German anatomist Johann Georg Wirsung discovered the pancreatic duct after performing a dissection on a man who had been hanged for murder. He named it the “duct of Wirsung” after himself, which may have upset some people. Wirsung was murdered the following year, allegedly over a disagreement as to who had actually discovered the duct.
4. It functions as both an endocrine and exocrine gland.
Although food never enters the pancreas, the organ does play a key role in digestion. It produces pancreatic fluid, which gets piped through the pancreatic duct to the duodenum. Once it’s in the digestive tract, the enzymes in the fluid help break down fat, protein, and carbohydrates. By sending a substance through ducts to other parts of the body, it functions as an exocrine gland. At the same time, it also functions as an endocrine gland by secreting two hormones directly into the bloodstream to help control blood sugars. Insulin is released when you have too much sugar, and glucagon is released when you don’t have enough sugar.
5. The pancreas can “taste” sugar.
The pancreas has taste receptor cells that let it sense the presence of sugar. It can “taste” artificial sweeteners, too. However, unlike the taste buds on our tongue, it doesn’t relay these sensations back to the brain. Instead, this sensory information helps the pancreas balance out the hormones and maintain healthy glucose levels in the body.
6. Diabetes is the result of damage to pancreatic cells.
For reasons that remain a scientific mystery, people with type 1 diabetes have immune systems that attack the insulin-producing cells in their pancreas. This prevents the cells from making insulin, and without insulin, other cells can't access the glucose in the bloodstream for energy. Sugar then builds up unhealthily in the bloodstream. People with type 2 diabetes, on the other hand, can still produce some insulin, but it’s not enough. Their cells become resistant to insulin (often as a result of obesity), which causes glucose to accumulate in the bloodstream.
7. The pancreas can digest itself.
Pancreatitis refers to the inflammation of the pancreas, but more alarmingly, what’s actually happening is that the digestive enzymes in the gland are going rogue and “digesting the pancreas itself,” according to Medline Plus. Heavy alcohol consumption is the most common cause of the disease, but other causes may include gallstones, cystic fibrosis, or high levels of fats or calcium in the blood. Most people with acute pancreatitis end up in the hospital, and it often goes away in a couple of days. Chronic pancreatitis can result in more serious complications.
8. Scorpion stings can cause pancreatitis.
The venom of a Brazilian scorpion, Tityus serrulatus, can cause pancreatitis, according to researchers at North Carolina State University. One particular enzyme in the venom attacks certain proteins in the gland, which impairs the pancreatic cells' functions and leads to inflammation. In a separate study of a related species (T. stigmurus), researchers found that “acute pancreatitis due to scorpion is usually transient [and] self-limited ... but it could progress to hemorrhagic pancreatitis and lead to death.”
9. Ruth Bader Ginsburg beat the odds and survived pancreatic cancer.
Ten years after she recovered from colon cancer, Ruth Bader Ginsburg received bad news following a routine check-up in 2009: She had pancreatic cancer. Fortunately, surgeons were able to remove the tumor, and at 85 years old (and counting), Ginsburg is now the oldest Justice on the U.S. Supreme Court. However, most people with pancreatic cancer aren’t so lucky. Although it’s less prevalent than skin, breast, and prostate cancers, it’s one of the deadliest. Just 8 percent of pancreatic cancer patients in the U.S. live longer than five years, according to the American Cancer Society.
James Cleary, an oncologist at the Dana-Farber Cancer Institute in Boston, says it’s very hard to catch in the early stages. “The reason pancreatic cancer can be so difficult to catch is number one, it’s a fast-moving cancer and can grow very rapidly,” he tells Mental Floss. “And number two, it can grow in a spot where you don’t get any symptoms until it’s too late.” In some cases, the cancer may start in the pancreas and spread to the liver or lining of the abdomen without any symptoms showing up.
10. Pancreatic surgery is extremely difficult to pull off.
Sometimes, patients with pancreatic cancer will undergo a complicated surgery called a Whipple procedure, which involves the removal of the head of the pancreas, part of the small intestine, the gallbladder and bile duct, and sometimes part of the stomach, too. However, very few people with pancreatic cancer are candidates for surgery—even if the cancer hasn’t yet spread to neighboring organs. That’s because cancer cells sometimes surround important blood vessels, making it “a tricky area” to operate on, according to Cleary. “The pancreas plays a really important role in digestion, and because of that, it’s very close to several important blood vessels and it’s very close to the stomach and small intestine,” he says.
11. There are genetic components to pancreatic cancer.
More than 90 percent of pancreatic cancers involve a mutation of the KRAS gene, which is also responsible for about half of all human cancers, according to Cleary. However, a drug hasn’t been invented yet to turn this particular gene off. “Finding a way to make a drug successfully target KRAS is one of holy grails of oncology," Cleary says. "It is of such great importance to oncology that a Nobel Prize could be awarded to whoever figures out how to make effective KRAS targeted therapy."
Mutations of DNA repair genes occur in up to 20 percent of pancreatic cancer cases. Some of these mutated genes, like BRCA1 and BRCA2, can run in families. This is why some families have several members who end up suffering from pancreatic cancer. Jimmy Carter, for example, lost his father, brother, and two sisters to pancreatic cancer. His mother had breast cancer that migrated to her pancreas. PARP inhibitors (drugs that block a particular enzyme) have been used to target DNA repair genes in breast and ovarian cancers, and there is now hope that they may also be effective in treating pancreatic cancer.
12. An aggressive form of chemotherapy is helping pancreatic cancer patients live longer.
A chemotherapy regimen called FOLFIRINOX has made significant improvements in the care of pancreatic cancer patients ever since it was introduced in 2010 as a treatment for patients with metastatic disease. Before 2010, “It was very, very rare to see anyone with metastatic cancer living longer than one year,” Cleary says. With FOLFIRNOX, it's not uncommon to see patients with metastatic pancreatic cancer living two years. A huge step forward came in June 2018 when researchers from France found that giving FOLFIRINOX after surgery could increase survival by a median of 20 months longer compared to the standard chemotherapy. Now, researchers are conducting trials to see if FOLFIRINOX can effectively be administered before a patient undergoes surgery. Considering that most patients aren’t eligible for surgery at diagnosis, pre-operative FOLFIRINOX could shrink the pancreatic tumor and increase the number of patients that are able to safely receive surgery.